Researchers at the University of Córdoba in Spain have developed a method of integrating hemoglobin, the primary constituent of red blood cells, into a battery. This innovation has resulted in the creation of a prototype that remained functional for a period of 20 to 30 days.
Zinc-air batteries provide a very sustainable alternative to the widely used lithium-ion batteries, which now provide power for a wide range of devices, including laptops and electric cars. They operate through a chemical process known as the oxygen reduction reaction. During the battery operation, the cathode (positive end) facilitates the reduction of oxygen to water, while the anode (negative end) facilitates the oxidation of zinc, resulting in the release of electrons.
In order to sustain the reaction, it is necessary to own a proficient catalyst that has very specialized features. The researchers discovered that hemoglobin had particular properties. “In order to effectively catalyze the oxygen reduction reaction, the catalyst must possess two essential properties: rapid absorption of oxygen molecules and facile formation of water molecules,” explained senior scientist Manuel Cano Luna in a formal statement. “Hemoglobin satisfied those criteria.”
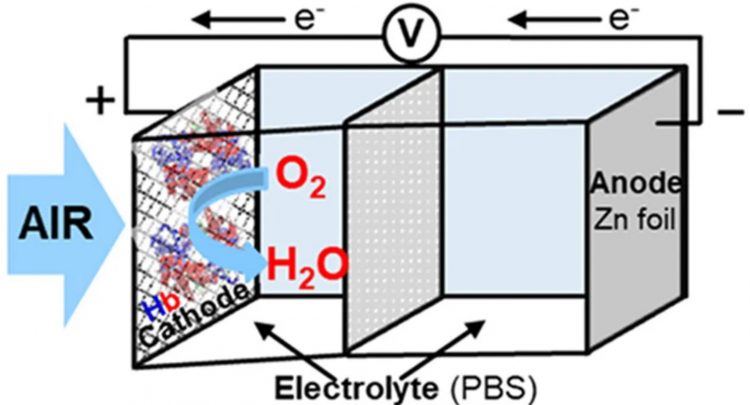
Hemoglobin is the protein responsible for the distinctive color and oxygen transport capacity of red blood cells. Hemoglobin, which is essential for human bodily functions, also shown remarkable performance in the battery, as a mere 0.165 milligrams of it sustained the battery’s operation for a period of 20-30 days.
According to the researchers, the use of a biocompatible catalyst like this is crucial for the application of these batteries in internally implanted devices, such as pacemakers. The battery operates optimally at a pH of 7.4, a level closely resembling the pH of blood. The potential applications might also include other species, since hemoglobin analogues are found in several animals.
Nevertheless, there are still certain aspects that need further refinement. The primary concern now is on the lack of rechargability in the prototype. Consequently, the team is actively seeking a protein with the potential to convert water into oxygen, thereby initiating a new cycle of reactions. The need for oxygen is a significant constraint, rendering these batteries unsuitable for use in space applications.
However, it remains an intriguing prospect. The challenge of energy storage continues to be a significant obstacle in humanity’s endeavor to transition towards a more environmentally friendly future. The performance of batteries is constantly improving. Although lithium-ion batteries clearly play a crucial role in this narrative, concerns around the availability of sufficient lithium and the resulting waste production indicate the need for other solutions.
One potential option may be a hemoglobin-based battery that is compatible with living organisms.
The research is published in the Energy & Fuels journal.
 Tech Gadget Central Latest Tech News and Reviews
Tech Gadget Central Latest Tech News and Reviews




